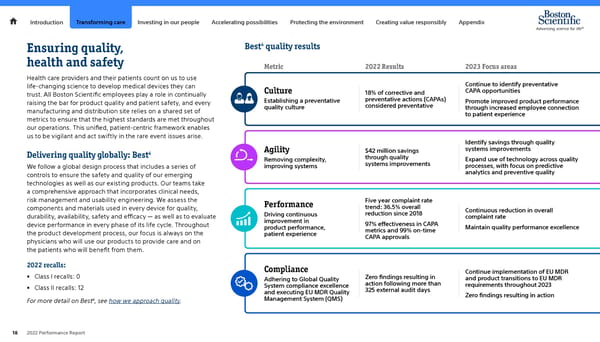
Introduction Transforming care Investing in our people Accelerating possibilities Protecting the environment Creating value responsibly Appendix 4 Ensuring quality, Best quality results health and safety Metric 2022 Results 2023 Focus areas Health care providers and their patients count on us to use life-changing science to develop medical devices they can Continue to identify preventative trust. All Boston Scientific employees play a role in continually Culture 18% of corrective and CAPA opportunities raising the bar for product quality and patient safety, and every Establishing a preventative preventative actions (CAPAs) Promote improved product performance quality culture considered preventative through increased employee connection manufacturing and distribution site relies on a shared set of to patient experience metrics to ensure that the highest standards are met throughout our operations. This unified, patient-centric framework enables us to be vigilant and act swiftly in the rare event issues arise. Identify savings through quality 4 Agility $42 million savings systems improvements Delivering quality globally: Best Removing complexity, through quality Expand use of technology across quality We follow a global design process that includes a series of improving systems systems improvements processes, with focus on predictive controls to ensure the safety and quality of our emerging analytics and preventive quality technologies as well as our existing products. Our teams take a comprehensive approach that incorporates clinical needs, risk management and usability engineering. We assess the Performance Five year complaint rate components and materials used in every device for quality, trend: 36.5% overall Continuous reduction in overall durability, availability, safety and efficacy — as well as to evaluate Driving continuous reduction since 2018 complaint rate device performance in every phase of its life cycle. Throughout improvement in 97% effectiveness in CAPA product performance, metrics and 99% on-time Maintain quality performance excellence the product development process, our focus is always on the patient experience CAPA approvals physicians who will use our products to provide care and on the patients who will benefit from them. 2022 recalls: Compliance • Class I recalls: 0 Continue implementation of EU MDR Adhering to Global Quality Zero findings resulting in and product transitions to EU MDR • Class II recalls: 12 System compliance excellence action following more than requirements throughout 2023 and executing EU MDR Quality 325 external audit days Management System (QMS) Zero findings resulting in action 4, see how we approach quality. For more detail on Best 18 2022 Performance Report
 Advancing Science for Life | Boston Scientific Page 17 Page 19
Advancing Science for Life | Boston Scientific Page 17 Page 19