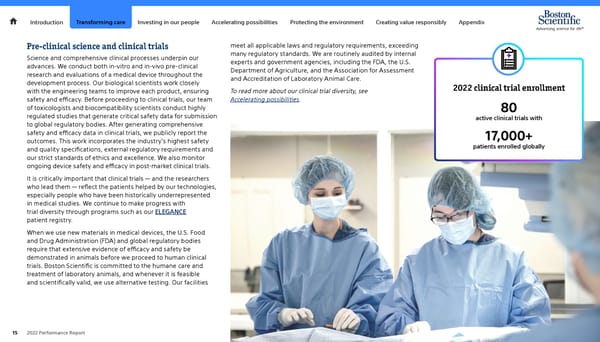
Introduction Transforming care Investing in our people Accelerating possibilities Protecting the environment Creating value responsibly Appendix Pre-clinical science and clinical trials meet all applicable laws and regulatory requirements, exceeding Science and comprehensive clinical processes underpin our many regulatory standards. We are routinely audited by internal advances. We conduct both in-vitro and in-vivo pre-clinical experts and government agencies, including the FDA, the U.S. research and evaluations of a medical device throughout the Department of Agriculture, and the Association for Assessment development process. Our biological scientists work closely and Accreditation of Laboratory Animal Care. with the engineering teams to improve each product, ensuring To read more about our clinical trial diversity, see 2022 clinical trial enrollment safety and efficacy. Before proceeding to clinical trials, our team Accelerating possibilities. of toxicologists and biocompatibility scientists conduct highly 80 regulated studies that generate critical safety data for submission active clinical trials with to global regulatory bodies. After generating comprehensive safety and efficacy data in clinical trials, we publicly report the 17,000+ outcomes. This work incorporates the industry's highest safety and quality specifications, external regulatory requirements and patients enrolled globally our strict standards of ethics and excellence. We also monitor ongoing device safety and efficacy in post-market clinical trials. It is critically important that clinical trials — and the researchers who lead them — reflect the patients helped by our technologies, especially people who have been historically underrepresented in medical studies. We continue to make progress with trial diversity through programs such as our ELEGANCE patient registry. When we use new materials in medical devices, the U.S. Food and Drug Administration (FDA) and global regulatory bodies require that extensive evidence of efficacy and safety be demonstrated in animals before we proceed to human clinical trials. Boston Scientific is committed to the humane care and treatment of laboratory animals, and whenever it is feasible and scientifically valid, we use alternative testing. Our facilities 15 2022 Performance Report
 Advancing Science for Life | Boston Scientific Page 14 Page 16
Advancing Science for Life | Boston Scientific Page 14 Page 16